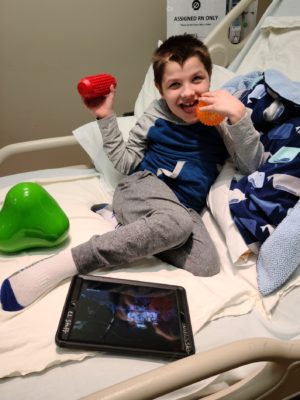
At the CHOC Research Institute, we know a rare disease diagnosis can be devastating for families. Our work focuses on developing and improving treatments for children with rare conditions that either do not respond to traditional treatments or that do not have current treatments.
Our innovative research in rare diseases spans from our Metabolic Rare Disease Program (MRDP) – which comprises about 80% of the pediatric rare diseases we encounter at CHOC – to rare diseases in hematology, neurology, oncology and neuro-oncology, cardiology, pulmonology, infectious disease, gastroenterology, critical care and urology.
Metabolic Rare Disease Research at CHOC
Our Metabolic Rare Disease Program (MRDP) is among the first comprehensive programs in the U.S. to offer the full continuum of services for children with rare metabolic conditions. Patients and their families have direct access to board-certified specialists, nurse practitioners, dietitians, genetic counselors, nurses and social workers, as well as diagnostic and research laboratory scientists, PhDs and doctoral students who can provide the latest therapies, including clinical trials. Our program advances research and education, and streamlines access to inpatient and outpatient services, resulting in better outcomes for children with rare diseases.
All our research activities are focused on rare or ultra-rare diseases and include:
- Adrenoleukodystrophy (ALD): ALD is a metabolic disease that can be devastating for patients and families if not identified and treated early. Since the start of the State of CA Newborn Screening Program, we have established a multidisciplinary clinic for clinical care and research that includes pediatric metabolic, neurology and endocrinology experts, as well as genetic counselors, case managers and social workers.
- Glycogen Storage Disease (GSD)
- Inborn Errors of Metabolism (IEM) Clinic
- Lysosomal Storage Diseases (LSD)
- Multidisciplinary Mucopolysaccharidosis Center
- Neurometabolic Disorders
- Newborn Screening: The MRDP program is the designated Orange County center for the evaluation and treatment of children with a presumed metabolic disease through the State of California Newborn Screening Program. MRDP has been instrumental in developing guidelines for the confirmatory testing of these conditions and has published extensively in this subject.
- Phenylketonuria (PKU)
Latest Metabolic Research
Outcomes in 14 live births resulting from Pegvaliase-treated pregnancies in PKU-affected females
Severe CNS involvement in a subset of long-term treated children with infantile-onset Pompe disease
Base editing corrects the common Salla disease <em>SLC17A5</em> c.115C>T variant
Growth patterns in patients with mucopolysaccharidosis VII
CHOC and UCI Health Join New NORD Rare Disease Centers of Excellence Network
Children’s Hospital of Orange County (CHOC) and University of California, Irvine (UCI) Health have been jointly designated a National Organization for Rare Disorders (NORD) Rare Disease Center of Excellence, joining a new and highly select group of 31 medical centers seeking to expand access and advance care and research for rare disease patients in the United States. The NORD Center of Excellence program aims to foster the sharing of knowledge between experts across the country, connect patients to appropriate specialists regardless of disease or geography, and improve the pace of progress in rare disease diagnosis, treatment, and research.
Innovative Metabolic Rare Disease Care at CHOC
The Metabolic Disorders team has diagnosed and cared for more than 800 families. Although the expansion of the California Newborn Screening Program has allowed more than 50% of our patients to be detected at birth, the remainder still rely on the expertise of the metabolic team for clinical evaluation and treatment. Our expert team of four board-certified metabolic specialists, nurse practitioners, metabolic dietitians, genetic counselors, case managers and social workers provide comprehensive and innovative care, making CHOC the leading destination for children with rare metabolic disorders.
CHOC Rare Disorder Patient Clinics
Our division leads multidisciplinary clinics alongside other pediatric specialties, offering a unique coordinated experience for our families. All clinics evaluate each family for enrollment in national registries, clinical trials or research protocols at CHOC, or other centers as appropriate, with the support of three clinical research coordinators. Additionally, our bench researchers (PhDs, scientists and technicians) frequently attend these clinics to interact with families and learn about their conditions firsthand.
CHOC Metabolic Laboratory
CHOC is one of the few pediatric hospitals in the nation with an in-house metabolic laboratory featuring state-of-the-art equipment. Testing capabilities include organic acid, amino acid, acylcarnitines and carnitine analyses, as well as the monitoring of metabolites for selected aminoacid disorders. The metabolic laboratory has recently expanded its services for the growing population of CHOC patients with speech delay, autism, movement disorders and seizures by offering testing for defects of creatine biosynthesis and vitamin B6 metabolism. The lab also supports clinical and basic science research projects, to monitor response to novel clinical or experimental treatments.
Scientific Rare Disease Research at CHOC
- Lysosomal Storage Disease Laboratory: The Lysosomal Storage Disease Laboratory (LSD-Lab) aims to develop more effective treatments for lysosomal storage disorders, beyond the currently available hematopoietic stem cell transplantation (HSCT) and intravenous enzyme replacement therapy (ERT). Specific projects include:
- Mucopolysaccharidosis (MPS): Research focuses on defining biomarkers that cause progression or improvement of cardiovascular disease. We have also advanced projects to study the use of combined intra-articular/intravenous/intracranial gene therapy for MPS I disease.
- Pompe Disease: We are utilizing cutting edge CRISPR genome editing to permanently correct the severe myopathy in Pompe disease mouse models
- Laboratory for Energy Metabolism (E-Lab): The E-Lab studies diseases affecting mitochondrial energy production. The E-Lab’s state-of-the-art equipment includes a bioinformatics workstation used for the analysis of whole exome, whole genome and whole transcriptome sequencing; tissue culture facilities; a hypoxia environmental incubation system; a SeaHorse bioanalyzer; a fluorescent microscope; a fluorescence-activated cell sorter; and others. These sophisticated pieces of equipment are used to uncover new gene defects, characterize diseases at the cellular level and investigate new therapies. Current projects include:
- DARS2 defects: Defects in the DARS2 gene cause Leukoencephalopathy with Brain Stem Involvement and Lactic acid elevation (LBSL). This is a mitochondrial disorder for which there is currently no effective treatment.
- Mitochondrial DNA depletion syndromes: E-lab research is focused on understanding why different organs (i.e. brain or liver) are selectively affected in some of these diseases. We are also exploring the metabolism of a co-factor (BH4) in these disorders.
- The Laboratory for Cellular Models of Disease (CM-Lab): The CM-Lab specializes in differentiating patient skin cells into pluripotent stem cells, which can then be further differentiated into organ-specific cell types (i.e. neurons). These newly generated cells are used to target tissue specific treatments.
- The Center for Advancing Rare disease Editing (CARE): This Center uses genome-editing technology to generate, characterize and treat animal models with rare diseases featuring known pathogenic mutations. The CARE laboratory has created the first mouse model for Pompe Disease (PD) and is applying the same principles of CRISPR-Cas9 technology to study Sialic Acid Storage Disorders (SASD).
- The Drug and Cell Discovery Laboratory (DC-Lab): The DC-Lab aims to develop therapies for rare disorders. Current projects include the use of cell culture and cell sorting systems to select cells for transplantation in the treatment of lysosomal Storage Disorders and to explore small-molecule (drug) approaches for mitochondrial disease therapy.
- Gene Therapy
- Gene Therapy Clinical Trial for Children With GM1 Gangliosidosis – Dr. Raymond Wang
- Gene Therapy Clinical Trial for Patients With Mucopolysaccharidosis Type I – Dr. Raymond Wang
- Gene Therapy Clinical Trial for Patients With Mucopolysaccharidosis Type IIIA – Dr. Raymond Wang
- Early Access Program for Arimoclomol for Patients with Niemann Pick C Disease – Dr. Raymond Wang
- Gene Therapy Clinical Trial for Patients with Phenylalanine Hydroxylase Deficiency (aka Phenylketonuria) – Dr. Richard Chang
- Gene therapy Clinical Trial for Patients with Glycogen Storage Disease Type Ia: (DTX401-CL301) – Dr. Jose Abdenur
- Cell Therapy: In collaboration with the CHOC Division of Hematology, the MRDP has also been a pioneer in cell therapy clinical trials, specifically hematopoietic stem-cell therapies (HSCTs) for metabolic diseases. This type of treatment has been applied at CHOC since the early 2000s for lysosomal diseases, like MPS type I, as well as peroxisomal diseases, such as X-linked adrenoleukodystrophy.
- Drug Treatments: The Division of Metabolic Disorders is a pioneer in the systematic evaluation and implementation of novel drug treatments. New projects include:
- The use of Arimoclomol for Niemann Pick type C and a new therapy for MPS type VII (UX007 Phase III Pivotal Trial- NCT02230566).
- Enzyme Therapy for Patients with Arginemia – Dr. Richard Chang
- The Neurometabolic team has also developed and published on new treatments to limit the accumulation of toxic metabolites in ultra-rare diseases.
Rare Disease Stories
A deep brain stimulation surgery at CHOC has given 9-year-old Ryder and his family an improved quality of life.
Though facing a rare disease, Oliver and his family found a diagnosis and hope at CHOC through the help of rapid whole-genome sequencing.
CHOC is the largest Brineura infusion center in the US to treat Batten disease, a rare neurological condition.